First described in 1967, bronchopulmonary dysplasia (BPD) is the most common cause of lung disease that afflicts premature neonates. BPD is defined as oxygen dependency at 36 weeks corrected gestational age[1]. This disease causes an arrest of the normal lung development characterized by decreased alveolarization, septation and microvasculature. Factors that increase the risk of developing BPD include preterm birth, intrauterine inflammation, and infections (e.g., clinical or sub-clinical chorioamniotis)[2, 3].
 Babies suffering from BPD present with abnormally rapid breathing (tachypnoe), abnormally rapid heart rate (tachycardia), increased respiratory effort, frequent episodes of hypoxia (desaturations), difficulty breathing (dyspnea), problems with feeding and swallowing, increased blood pressure in the pulmonary arteries, and failure of the right side of the heart [4-6].
Babies suffering from BPD present with abnormally rapid breathing (tachypnoe), abnormally rapid heart rate (tachycardia), increased respiratory effort, frequent episodes of hypoxia (desaturations), difficulty breathing (dyspnea), problems with feeding and swallowing, increased blood pressure in the pulmonary arteries, and failure of the right side of the heart [4-6].
- Carraro, S., Filippone, M., Da Dalt, L., Ferraro, V., Maretti, M., Bressan, S., El Mazloum, D., Baraldi, E. (2013). Bronchopulmonary dysplasia: the earliest and perhaps the longest lasting obstructive lung disease in humans. Early Hum Dev, 89 Suppl 3:S3-5.
- Jobe, A. H. and Bancalari, E. (2001). Bronchopulmonary dysplasia. Am J Respir Crit Care Med, 163(7):1723-1729.
- Jobe, A. H. (2012). What is BPD in 2012 and what will BPD become? Early Hum Dev, 88 Suppl 2: S27-28.
- Jain, D. and Bancalari, E. (2014). Bronchopulmonary Dysplasia: Clinical Perspectives. Birth Defects Research (Part A), 100:134-144.
- Ruffini, L. (2015). Pulmonary hypertension in a premature infant with bronchopulmonary dysplasia. J Pediatr Health Care.
- An, H. S., Bae, E. J., Kim, G. B. Kwon, B. S., Beak, J. S., Kim, E. K., Kim, H. S., Choi, J. H., Noh, C. I., Yun, Y. S. (2010). Pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. Korean Circ J, 40(3):131-136.
- The lungs of premature infants are not fully formed and lack surfactant and its proteins, including surfactant protein D (SP-D).
- 80% of infants born before 27 weeks gestation need respiratory support to live[1].
- Respiratory support involves treatment with commercial surfactant (which lacks SP-D), supplemental oxygen and breathing assistance from a mechanical ventilator.
- The ventilator and oxygen support preserve life, but damage the delicate lung tissue, and commercial surfactant alone cannot protect against the damage.
- The fragile lungs react to the damage by becoming inflamed, causing susceptibility to infection and disease.
- Inflammation also arrests lung development, leading to formation of immature gas exchange units.
- The result is bronchopulmonary dysplasia (BPD)[2-6].
- Stoll, B. J., Hansen, N. I., Bell, E. F., Shankaran, S., Laptook, A. R., Walsh, M. C., Hale, E. C., Newman, N. S., Schibler, K., Carlo, W. A., Kennedy, K. A., Poindexter, B. B., Finer, N. N., Ehrenkranz, R. A., Duara, S., Sanchez, P. J., O’Shea, M., Goldber, R. N., Van Meurs, K. P., Faix, R. G., Phelps, D. L., Frantz III, I. D., Watterberg, K. L., Saha, S., Das, A., & Higgins, R. D. (2010). Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics, 126(3), 443-456. doi: 10.1542/peds.2009-2059
- Halliday, H. L., Ehrenkranz, R. A., and Doyle, L. W. (2010). Early (< 8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database Syst Rev, Jan 21;(1):CD001146. doi: 10.1002/14651858.CD001146.pub2.
- Popova, A.P. (2013) Mechanisms of bronchopulmonary dysplasia. J Cell Commun Signal, 7(2): 119-27.
- Wright, C. J. and Kirpalani, H. (2011). Targeting inflammation to prevent bronchopulmonary dysplasia: can new insights be translated into therapies? Pediatrics, 128(1): 111-26.
- Speer, C. P. (2006). Inflammation and bronchopulmonary dysplasia: a continuing story. Semin Fetal Neonatal Med, 11(5):354-62.
- Bhandari, V. (2014) Postnatal inflammation in the pathogenesis of bronchopulmonary dysplasia. Birth Defects Res A Clin Mol Teratol, 100(3): 189-201.
- Breathing difficulties (dyspnea)
- Problems feeding and swallowing
- Increased blood pressure in the pulmonary arteries (pulmonary hypertension)
- Failure of the right side of the heart
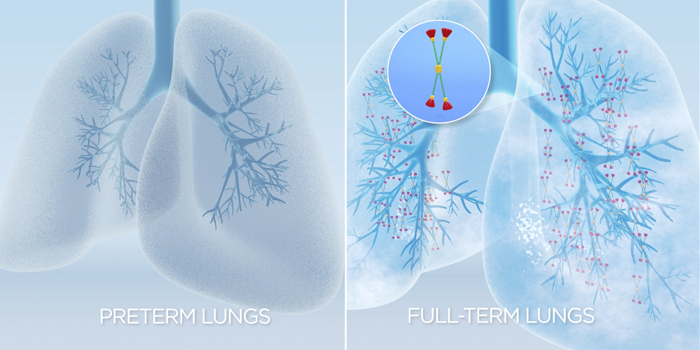
Surfactant Protein-D and Lung Development
The lungs of premature infants are not fully developed and lack pulmonary surfactant and reasonable levels of its associated proteins, including surfactant protein-D (SP-D). When a baby is born very prematurely, the lungs have not yet begun to make pulmonary surfactant. Without surfactant, the babies require assistance in order to breathe because the surfactant decreases lung compliance, allowing the lungs to expand and contract against the pressure of the chest wall. In a mature lung, SP-D prevents inflammation and infections by its immunomodulatory properties and is involved in surfactant pool recycling. This enables the surfactant to be more efficiently taken up by the cells in the alveoli where it is synthesized and secreted[4, 5]. While detectable at birth in preterm babies, SP-D levels rise significantly during the early postnatal period[6]. Up to 80% of infants that are born prior to 27 weeks gestational age require respiratory support in order to live[7].
Up to 80% of infants that are born prior to 27 weeks gestational age require respiratory support in order to live.
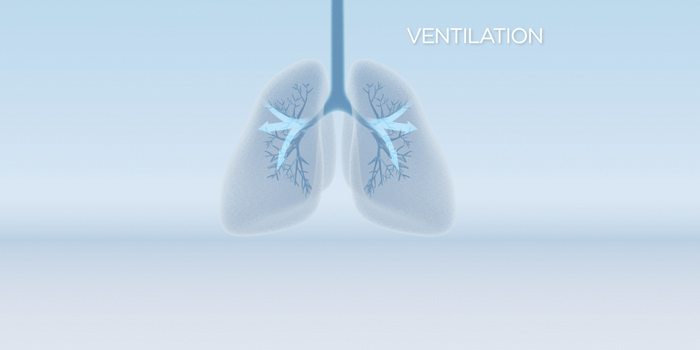
Impact of Ventilation
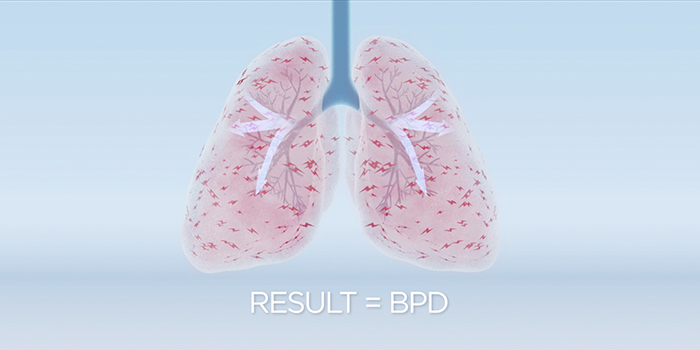
Respiratory support involves treatment with commercial surfactant (which lacks SP-D), supplemental oxygen, and breathing assistance from a mechanical ventilator. Although the ventilation and oxygen support preserve life, they damage the neonate’s delicate lung tissue, and commercial surfactant alone cannot protect against this damage. The fragile lungs react to this damage by becoming inflamed, which causes scarring and susceptibility to infections and disease. Inflammation also arrests lung development, leading to formation of immature gas exchange units. The result is BPD[8-11].
- Jain, D. and Bancalari, E. (2014). Bronchopulmonary Dysplasia: Clinical Outcomes. Birth Defects Research (Part A), 100:134-144.
- Ruffini, L. (2015). Pulmonary hypertension in a premature infant with bronchopulmonary dysplasia. J Pediatr Health Care. doi: 10.1016/j.pedhc.2015.03.003 epub.
- An, H. S., Bae, E. J., Kim, G. B. Kwon, B. S., Beak, J. S., Kim, E. K., Kim, H. S., Choi, J. H., Noh, C. I., Yun, Y. S. (2010). Pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. Korean Circ J, 40(3):131-136.
- Crouch, E. C. (2000). Surfactant protein-D and pulmonary host defense. Respir Res, 1(2):93-108.
- Kishore, U., Greenhough, T. J., Waters, P., Shrive, A. K., Ghai, R., Kamran, M. F., Bernal, A. L., Reid, K. B., Madan, T., Chakraborty, T. (2006). Surfactant proteins SP-A and SP-D: structure, function, and receptors. Mol Immunol, 43(9):1293-1315.
- Beresford, M. W. and Shaw, N. J. (2003). Bronchoalveolar lavage surfactant protein A, B, and D concentrations in preterm infants ventilated for respiratory distress syndrome receiving natural and synthetic surfactants. Pediatric Res, 53(4):663-670.
- Stoll, B. J., Hansen, N. I., Bell, E. F., Shankaran, S., Laptook, A. R., Walsh, M. C., Hale, E. C., Newman, N. S., Schibler, K., Carlo, W. A., Kennedy, K. A., Poindexter, B. B., Finer, N. N., Ehrenkranz, R. A., Duara, S., Sanchez, P. J., O’Shea, M., Goldber, R. N., Van Meurs, K. P., Faix, R. G., Phelps, D. L., Frantz III, I. D., Watterberg, K. L., Saha, S., Das, A., & Higgins, R. D. (2010). Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics, 126(3), 443-456. doi: 10.1542/peds.2009-2059
- Jobe, A. H. (2012). What is BPD in 2012 and what will BPD become? Early Hum Dev, 88 Suppl 2: S27-28.
- Hadchouel, A., Franco-Montoya, A. L., Delacourt, C., (2014). Altered lung development in bronchopulmonary dysplasia. Birth Defects Res A Clin Mol Teratol, 100(3), 158-167.
- Wright, C. J. and Kirpalani, H. (2011). Targeting inflammation to prevent bronchopulmonary dysplasia: can new insights be translated into therapies? Pediatrics, 128(1):111-126.
- Bhandari, V. (2014). Postnatal inflammation in the pathogenesis of bronchopulmonary dysplasia. Birth Defects Res A Clin Mol Teratol, 100(3):189-201.
Very low birth weight neonates (<32 weeks gestational age) are particularly at risk for this disease[1-3].
Prevalence of BPD
Airway Therapeutics has consulted with leading neonatologists and extensively reviewed published data in order to estimate the percentage of infants likely to use or benefit from AT-100 [1-16]. Airway estimates that up to 160,000 infants are at highest risk for the development of BPD in Europe and the United States, combined, each year. With 73.6 million live births in Asia and a preterm rate <32 weeks of approximately 2.2%, we estimate an addressable market of 1.6 million babies [16].
Why Target This Disease?
- More babies get BPD today than 30 years ago – the increase in incidence of preterm babies living longer is due to surfactant and ventilation applications
- BPD has lifelong consequences – it is associated with chronic lung disease (e.g. asthma, recurrent pneumonia), as well as growth and neurodevelopmental problems
- Tremendous burden to the medical resource utilization – estimated costs of BPD management in the US is over $2 billion annually [17-18]
- Fanaroff, A. A., Stoll, B. J., Wright, L. L., Carlo, W. A., Ehrenkranz, R. A., Stark, A. R., Bauer, C. R., Donovan, E. F., Korones, S. B., Laptook, A. R., Lemons, J. A., Oh, W., Papile, L., Shankaran, S., Stevenson, D. K., Tyson, J. E., & Poole, W. K. (2007). Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol, 196(2), 147e1-e8. doi: 10.1016/j.ajog.2006.09.014
- Shah, P. S., Sankaran, K., Allen, A. C., Seshia, M., Ohlsson, A., & Lee, S. K. (2012). Outcomes of preterm infants <29 weeks gestation over 10-year period in Canada: a cause for concern? Perinatology, 32(2), 132-138. doi: 10.1038/jp.2011.68.
- Stoll, B. J., Hansen, N. I., Bell, E. F., Shankaran, S., Laptook, A. R., Walsh, M. C., Hale, E. C., Newman, N. S., Schibler, K., Carlo, W. A., Kennedy, K. A., Poindexter, B. B., Finer, N. N., Ehrenkranz, R. A., Duara, S., Sanchez, P. J., O’Shea, M., Goldber, R. N., Van Meurs, K. P., Faix, R. G., Phelps, D. L., Frantz III, I. D., Watterberg, K. L., Saha, S., Das, A., & Higgins, R. D. (2010). Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics, 126(3), 443-456. doi: 10.1542/peds.2009-2059
- Astolfi, P., De Pasquale, A., Zonta, L.A. (2007). Gestational age shortening in single births at term. Italy 1990-1998. Eur J Epidemiol, 22(4): p. 263-265.
- Bergmann, R.L., Richter, R., Bergmann, K.E., & Dudenhausen, J.W. (2004). The prevalence of preterm deliveries in Berlin has not changed over 7 years: the impact of multiple births. J Perinat Med, 32(3): p. 234-239.
- Blondel, B., Kogan, M.D., Alexander, G.R., Dattani, N., Kramer, M.S., Macfarlane, A., & Wen, S.W. (2002). The impact of the increasing number of multiple births on the rates of preterm birth and low birthweight: an international study. Am J Public Health, 92(8): p. 1323-1330.
- Elder, M. (2012). Clinical nutrition products: a worldwide perspective. Kalorama.
- Hamilton, B. E., Martin, J. A., and Ventura, S. J. (2012). Births: preliminary data for 2011. National Vital Statistics Reports, 61(5).
- Isayama, T., Lee, S. K., Mori, R., Kusuda, S., Fujimura, M., Ye., X. Y., & Shah, P. S. (2012). Comparison of mortality and morbidity of very low birth weight infants between Canada and Japan. Pediatrics, 130(4), e957-965. doi: 10.1542/peds.2012-0336
- Jobe, A.H. and Bancalari, E. (2001). Bronchopulmonary Dysplasia. Am J Respir Crit Care Med 163(7), p. 1723-29
- Langhoff-Roos, J, Kesmodel, U, Jacobsson, B, Rasmussen, S, & Vogel, I. (2006). Spontaneous preterm delivery in primiparous women at low risk in Denmark: population based study. BMJ, 332(7547): p. 937-939.
- Lee, S.J., Hajat, S., Steer, P.J., & Filippi, V. (2008). A time-series analysis of any short-term effects of meteorological and air pollution factors on preterm births in London. UK. Environ Res, 106(2): p. 185-194.
- Morken, N.H., Källen, K., Hagberg, H., & Jacobsson, B. (2005). Preterm birth in Sweden 1973-2001: rate, subgroups, and effect of changing patterns in multiple births, maternal age, and smoking. Acta Obstet Gynecol Scand, 84(6): p. 558-565.
- Popova, A. P. (2013). Mechanisms of bronchopulmonary dysplasia. J Cell Commun Signal, 7(2), 119-127. doi: 10.1007/s12079-013-0190-x.
- Zeitlin, J., Szamotulska, K., Drewniak, N., Mohangoo, A., Chalmers, J., Sakkeus, L., Irgens, L., Gatt, M., Gissler, M., & Blondel, B. (2013). Preterm birth time trends in Europe: a study of 19 countries. BJOG, 120(11): p. 1356-1365.
- Blencowe, H., Cousens, S., Oestergaard, M. Z., Chou, D., Moller, A., Narwal, R., Adler, A., Garcia, C. V., Rohde, S., Say, L., & Lawn, J. E. (2012). National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet, 379(9832), 2162-2172. doi: 10.1016/S0140-6736(12)60820-4.
- Johnson, T. J., Patel, A. L., Bigger, H. R., Engstrom, J. L. , Meier, P. P. (2014). Economic benefits and costs of human milk feedings: a strategy to reduce the risk of prematurity-related morbidities in very-low-birth-weight infants. Adv Nutr, 5(2): 207-212.
- Landry, J. S., Croitoru, D., Jin, Y., Schwartzman, K., Benedetti, A., Menzies, D. (2012). Health care utilization by preterm infants with respiratory complications in Quebec. Can Respir J, 19(4):255-260.